新东方APP下载入口
新东方网整理2019-10-29 10:11新东方网
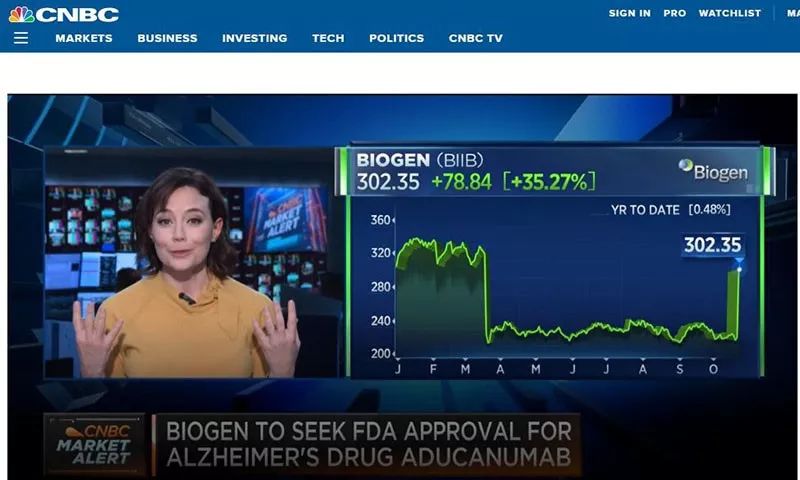
早在今年3月,百健和卫材公司曾宣布,将终止对“aducanumab”药物的两项后期试验,因为当时一项分析显示,他们成功研制出治疗阿尔茨海默综合症药物的希望十分渺茫。当时,这两家公司的股价一路大跌。
The announcement is somewhat surprising because the company had discontinued work on the drug in March 2019, after disappointing trial results.

不过,该公司称,新的数据显示,加大剂量对于治疗阿尔茨海默症早期症状,有显著效果,可以减缓患者记忆力衰退、遗忘日常生活技能等症状。
But the company says a new analysis of a larger dataset of the same studies shows that higher doses of aducanumab can provide a significant benefit to patients with early Alzheimer's, slowing their clinical decline so they preserve more of their memory and every day living skills - things that the disease usually robs.
根据最新试验分析,该公司“复活”了aducanumab。
The company resurrected the drug after additional analyses suggested it might have some effect at higher doses.百健此次宣布药物的消息令市场十分兴奋。据报道,22日,百健公司的股票价格飙升27%,卫材公司的股价上涨了18%。几乎收回了3月份放弃这两项研究时的损失。
对于这一消息,《纽约时报》直言,不能高兴太早(it is not quite time for these patients to celebrate)。
文章指出,该公司还没有公布最新分析数据,效果究竟如何还不得而知。目前看,只是能延缓阿尔茨海默症患者的一些认知衰退症状。
The company has not published the most recent analyses, and experts are mostly in the dark as to how well the drug works. It neither prevents nor cures Alzheimer's; the company claims only that aducanumab may slow cognitive decline in some patients.
该公司尚未公布最新分析数据,专家们大多数并不知道该药物效果如何。它不是预防或治愈阿尔茨海默症。公司只是说aducanumab可能会延缓某些患者的认知衰退症状。
相关推荐
- 美国最新疫情消息:美国新冠肺炎累计确诊超100万
- 双语:Pokemon Go怕玩家不隔离,要变成Pokemon Stay
- JK罗琳秘密买回哈利波特的诞生地
- 新东方APP下载入口
- 新东方APP下载入口
- 新东方APP下载入口